Posted on January 14, 2020
Will a ductile iron coupling on stainless steel pipe cause dissimilar metal corrosion?
Strength, corrosion resistance, and low cost of maintenance are all reasons that mechanical building services projects may call for the use of stainless steel pipe. But how should stainless steel pipe be joined together? And do I need to worry about dissimilar metal corrosion if I choose a grooved ductile iron coupling? No. In this blog post, we will explain how dissimilar metal corrosion occurs and why choosing a grooved mechanical pipe joining solution is ideal for joining stainless steel and copper piping systems.
What is Dissimilar Metal Corrosion?
Dissimilar Metal Corrosion is the electrochemical process that damages metal by reducing its strength and thickness. Dissimilar metal corrosion is also known as “Galvanic Corrosion” or “Bimetallic Corrosion.”
Three things that need to be present for galvanic corrosion to occur:
- Two dissimilar metals
- Electrolyte
- Return path
What is a dissimilar metal?
To understand dissimilar metals, you need to understand the Galvanic Series, which determines the nobility, or resistance to chemical reactions, of metals.
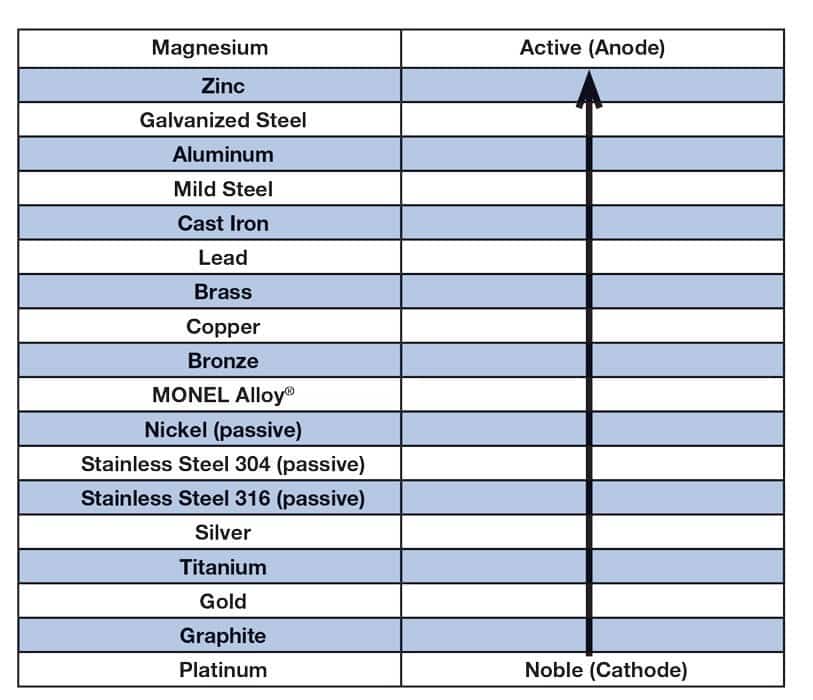
The Galvanic Series Chart lists metals in the order of their nobility. The metal that is more noble, and higher on the Galvanic Series chart, is called the cathode. The metal that is less noble, and lower on the Galvanic Series chart, is called the anode. Cathodic metals have a higher relative potential, or volts, than the anodic metals.
In dissimilar metal corrosion, the most severe attacks occur between metals that have greater differences in relative potentials. For example, titanium and aluminum would have a far greater or severe attack, in a dissimilar metal situation, than copper and brass would. This is due to the fact that titanium and aluminum have a greater difference in relative potentials when compare to copper and brass. (Which is illustrated by their distance from one another on the Galvanic Series chart.)
What is an electrolyte as it relates to metal corrosion?
To understand how and why the “attacks” occur between dissimilar metals, we will look at the flow of ions from one metal to another.
All metals have specific relative electrical potentials. When metals of different electrical potentials are in contact in the presence of an electrolyte, a low energy electrical current flows from the anodic metal to the cathodic metal. As previously mentioned, more noble metals are cathodic; metals that are less noble are anodic and are more likely to corrode relative to the cathodic metal that it is in contact with.
How to create a path for metal corrosion
When two dissimilar metals come into contact in a corrosive setting, the anodic metal undergoes galvanic corrosion while the cathodic metal maintains galvanic protection. This corrosion is created because the electrolyte provides a conduit for ion migration, moving metal ions from the anode to the cathode. The anode metal, as a result, corrodes more quickly than it otherwise would, while the cathode metal corrodes more slowly and, in some cases, may not corrode at all.
The image below is a representation of two metallic pipes made of dissimilar metals that are not joined properly, and have water flowing through them.
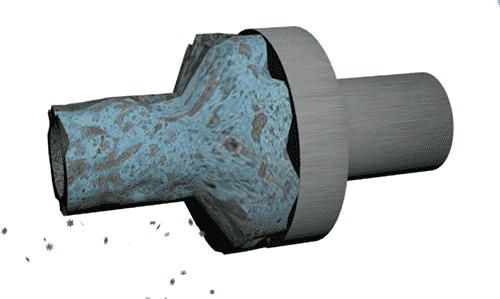
In this instance, the anodic metal is on the left. This metal is less noble, and the electrons are traveling from this metal to the cathodic metal through the pathway provided by the water. In other words, the water is the electrolyte transporting the metal ions from the anodic metal to the cathodic metal. Depending on the metal and the environment, the anodic metal will eventually corrode away in time. The dissimilar metal corrosion occurs because the two pipes were not electrically insulated from one another properly.
There are many instances where dissimilar metals are in contact with one another, and no corrosion occurs. An everyday example would be when you carry a copper penny and a nickel in your pocket. Using the Galvanic Series chart we see that copper is more anodic, or active, than nickel. We have two dissimilar metals that are in contact with one another; however, neither of the coins experience corrosion. That is because we are missing an electrolyte and a path.
The same concept of the nickel and penny your pocket applies to Victaulic products installed in a building services project. Some applications on these projects call for the use of stainless steel pipes. Stainless steel piping is most often utilized for its strength, corrosion resistance, and low cost of maintenance. Despite being dissimilar metals, couplings with ductile iron housings can be the best overall choice for joining stainless steel pipes.
The use of Victaulic couplings with ductile iron housings on stainless steel pipe is common practice when pipe material selection is based on compatibility with the fluid media and where the risk of external corrosion is low. Due to the design of the coupling housings and elastomer gasket, the housings will never contact the internal fluid media. The pressure-responsive gasket inside the joint provides a leak tight seal, completely isolating the fluid media within the interior of the pipe and gasket, and preventing contact with the coupling housings.
Victaulic has many years of jobsite experience with couplings with galvanized ductile iron housings installed on stainless steel piping systems without the report of galvanic corrosion between the pipe and couplings.
What happens when a corrosive setting is introduced?
Thinking back to the nickel and penny in your pocket, if by chance you jump into a swimming pool with the coins still in your pocket, then dissimilar metal corrosion will occur. This is because the three requirements, as previously discussed, are present in this situation:
- Dissimilar Metals: Nickel and Copper
- Electrolyte: Water
- Pathway: Electrons Travel via the Water
Can I use stainless steel couplings on stainless steel pipe?
Yes, you can use stainless steel couplings on stainless steel pipe; however, it can be costly and may not be necessary on some applications. Some projects will specify stainless steel piping because of the external environment surrounding the piping system. While the fluid media is isolated from contact with the coupling housings by the gasket, the pipe joint must be protected from exterior water.
Situations where exterior moisture can build-up and where the dissimilar metals are in contact include:
- pipe sweating
- buried applications
- submerged applications
In these situations, dissimilar metal corrosion will occur causing the galvanized ductile iron housings to show signs of surface corrosion similar to any ferrous metal when subjected to moist environments. If stainless steel piping has been chosen because of aggressive external environmental conditions surrounding the piping system, then the system engineer of record must evaluate the suitability of painted or galvanized couplings vs stainless steel couplings. For extremely corrosive external exposure, the system engineer may wish to use couplings with stainless steel housings and nuts & bolts.
Can ductile iron couplings be used on copper tube?
Yes. Victaulic has a long history (since 1988) of providing couplings with ductile iron housings for use on copper tube.
The Victaulic copper connection system provides a fast, easy, clean, reliable method for joining roll grooved copper tubing with no weld/solder flame, cutting oils or metal chips. Victaulic copper connection products are permitted for use on aboveground or underground potable water systems by national organizations such as International Association of Plumbers and Mechanical Officials (IAPMO) and International Plumbing Code (IPC).
Copper connection products have been successfully used in buried service applications for many years. Soil conditions in buried pipeline services must be examined to determine what corrosive effect they will have on the system components, including the coupling housings. The system designer has a responsibility to incorporate the appropriate protective coatings on the couplings and flange adapters to protect from galvanic corrosion and aggressive soil conditions such as ground water and soil acidity/alkalinity.
Victaulic couplings’ benefits address the problems of multiple negative environments and can provide noise and vibration control for HVAC systems. Click here to learn more.
Piping can be connected by both rigid and flexible couplings. But how are these couplings different? Click here to learn more about the history, differences, and applications of Victaulic flexible and rigid couplings.